

One of the leading causes of joint pain is a condition known as osteoarthritis, which can often result in marked impairment of mobility and quality of life in patients.
Those who are mainly affected are those over the age of 60. It is estimated that about 18% of women and 9.6% of men in this category suffer from a symptomatic form of OA and that 10% of the world’s population, which is 60 years old, have a significant health problem attributable to the disease.
However, often, to present the first symptoms of osteoarthritis are also the so-called young adults or subjects belonging to the age group between 35 and 50 years: in this case, we speak of early OA due, in general, to excessive effort or trauma related to sports or work.
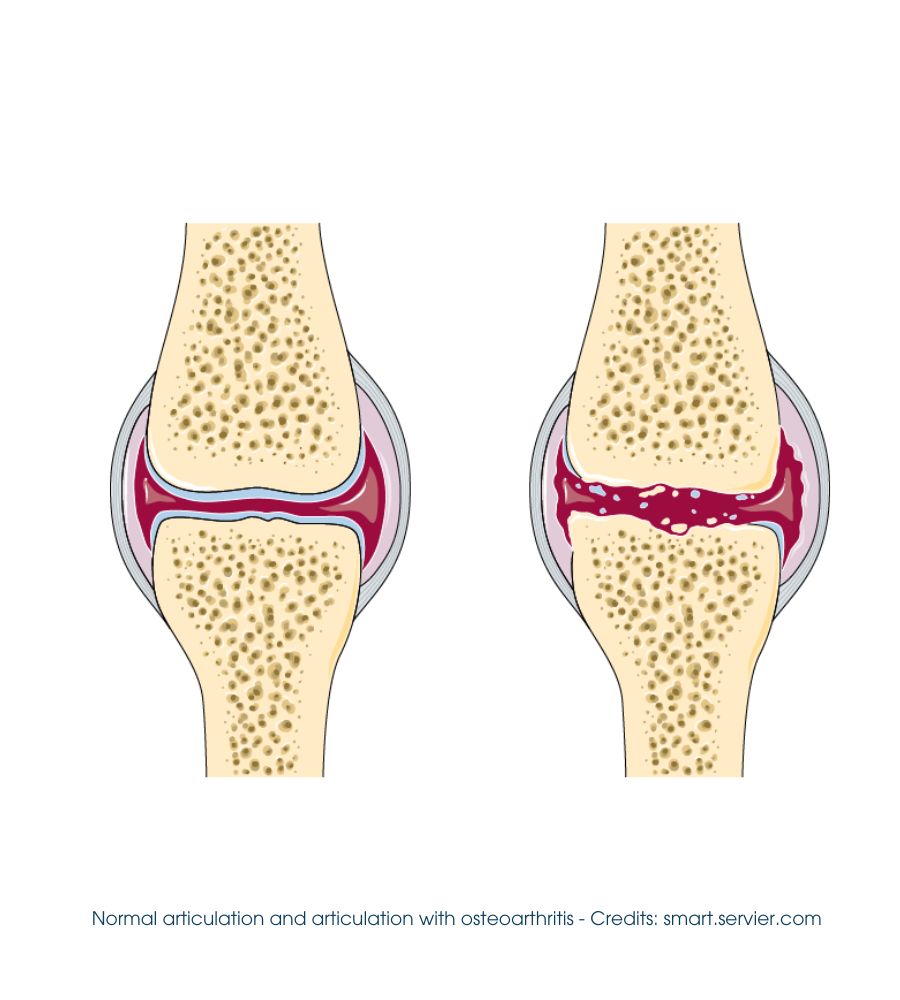
Osteoarthritis, also known as osteoarthritis or simply osteoarthritis, consists of the degenerative alteration of the articular cartilage, resulting in a remodeling of the adjacent bone and associated inflammation.
During the progression of OA, the endogenous hyaluronic acid present in the joint depolymerizes, passing from a high molecular weight (6500-10,900 kDa) to a lower molecular weight (2700-4500 kDa), with a consequent decrease in the mechanical and viscoelastic properties of the synovial fluid of the affected area.
The peripheral joints most commonly affected are the knee and hip, but the condition, in some cases, can also affect the small joints of the hand, feet, and spine.
At the initial stage, osteoarthritis is asymptomatic, as it affects the un-innervated cartilage. Still, when the lesion affects the surrounding tissues, the main signs and symptoms appear as pain, stiffness, and loss of movement and functionality.
The main causes or risk factors for the development of the disease are numerous, and their identification is vital for diagnosing the presence of the disease – especially in the case of early osteoarthritis – and for identifying therapeutic targets on which to act where possible.
Risk factors can be classified into modifiable and non-modifiable. In the first category, we find overweight, misalignment or dysmetria of the lower limbs, and excessive joint strain. While among the non-modifiable factors, we have age, gender, the presence of previous traumas to the affected joint, and the presence of comorbidities, that is, the coexistence of additional pathologies (such as metabolic syndrome) or familiarity.
Currently, there are no actual treatments for osteoarthritis. The applied remedies can only manage the symptoms and, in particular, relieve pain. Among these, there may be pharmacological, non-pharmacological solutions (for example, elimination of modifiable risk factors through a lifestyle change), which can be used in a complementary or alternative way. Finally, the surgical resolution of the problem should be reserved only for patients who do not improve with behavioral and pharmacological therapy and who present pain and loss of intractable function.
In this context, Visco supplementation emerges as an essential ally to alleviate the symptoms of OA and slow down its progression with injections of hyaluronic acid, which acts by helping to cushion and lubricate the moving parts within the joint area. In this sense, Vijoint, the product developed by the research and development laboratories of Biofarma Group, represents a valid example on the market.
The market of therapeutic drugs for osteoarthritis was valued at 7.3 billion dollars in 2020 and is expected to reach 11 billion dollars by 2028, with a growth rate of 8.7%.
To provide a good solution for osteoarticular support of subjects suffering from osteoarthritis, Biofarma Group has developed the Vijoint line, which includes 3 products with different concentrations of molecular weight of hyaluronic acid.
Within this line, we find Vijoint HCC, specifically, a class III medical device that acts as a temporary substitute for synovial fluid, helping to restore the physiological and rheological properties of joints reduced due to osteoarthritis.
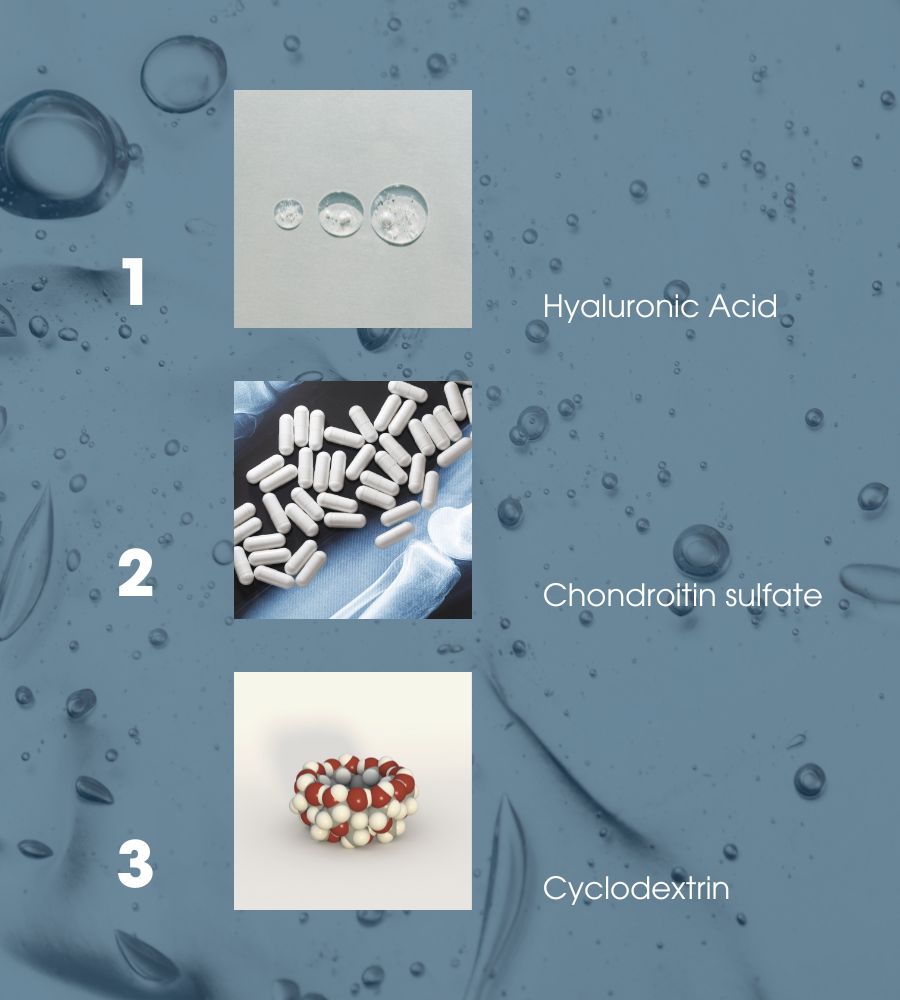
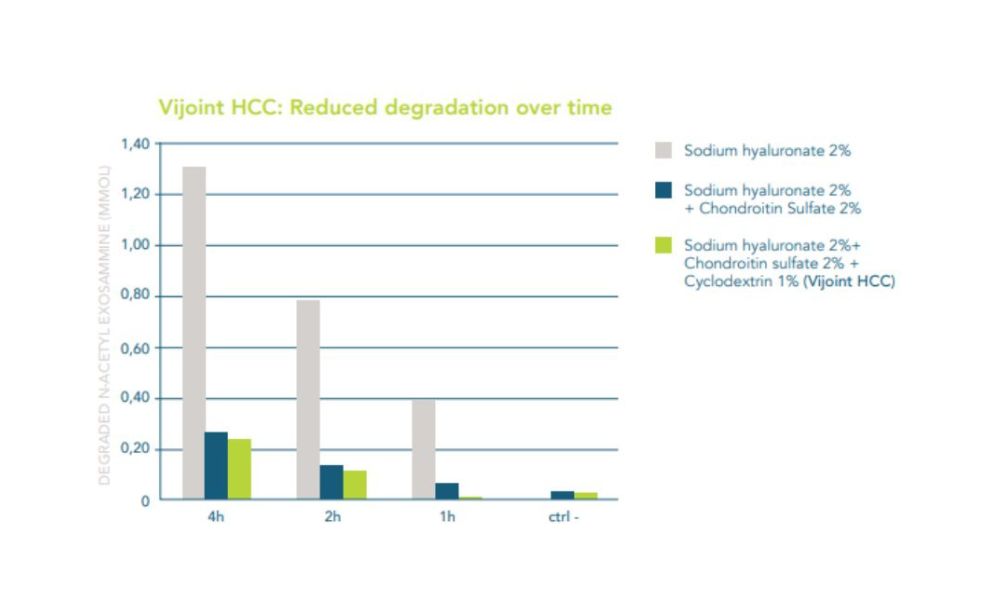
The main active ingredients of the composition are hyaluronic acid, chondroitin sulfate (of marine origin), and cyclodextrin.
Hyaluronic acid is a natural polysaccharide in many human tissues, particularly in synovial fluid, which acts in the joints as a lubricant of cartilage and ligaments, and as a shock absorber. According to numerous studies, when injected into the joints affected by osteoarthritis, it can restore the viscosity and elasticity of the synovial fluid, with a consequent attenuation of pain and an improvement in mobility. The hyaluronic acid in this device is obtained by fermentation and has not undergone a chemical transformation to guarantee excellent tolerability.
Chondroitin-sulfate is an essential component of articular cartilage, giving it much of its compressive strength. In addition to performing an antioxidant and anti-inflammatory function, in vitro, it has demonstrated the ability to inhibit the effect of enzymes that degrade hyaluronic acid, thus conferring more excellent resistance.
Finally, cyclodextrins are cyclic oligosaccharides able to improve the solubility in water of some substances, strengthening their stability and changing their state of aggregation from liquid to viscous. Thanks to the ability of these components, the solution of hyaluronic acid and chondroitin-sulfate shows physical characteristics such as guaranteeing better effectiveness.
VIjoint HCC is supplied in a glass syringe containing 3 ml of saline packed in blisters. The recommended use of the product is to be administered once per therapy, with the possibility, if necessary, to repeat the injection according to the condition of the individual patient and the evaluation of the competent physician.
Its effectiveness has been tested through a clinical study showing that the product is a safe, well-tolerated, and effective treatment to reduce osteoarticular pain and improve physical function in adults with OA.
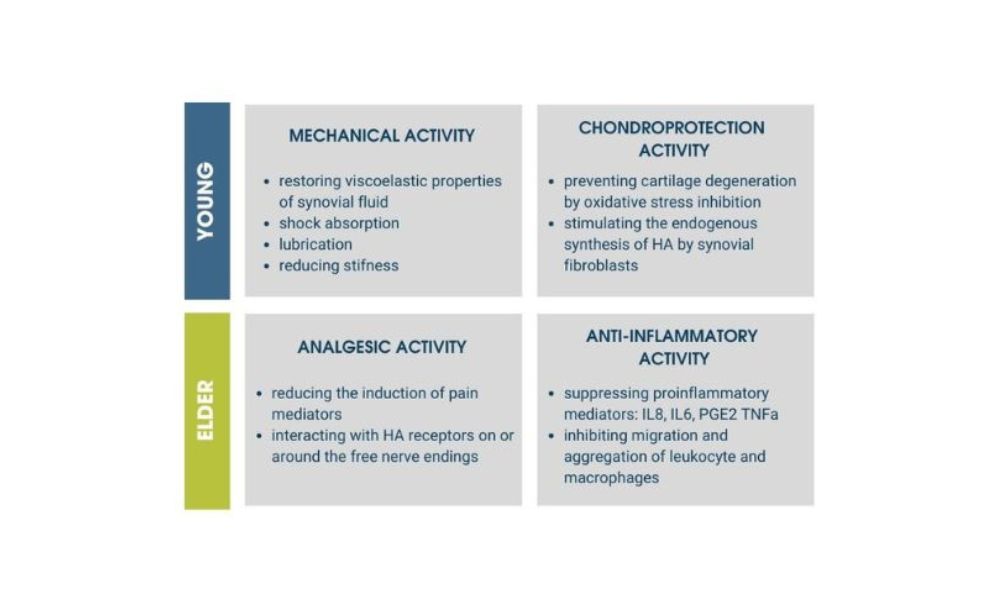
Specifically, it is a product with a dual function that allows you to enter the market by addressing a wide target in terms of age. On the one hand, it counteracts cases of early osteoarthritis, relieving pain, reducing inflammation, and improving joint function; on the other hand, it supports cases of osteoarticular decay due to advanced age, favoring the formation of subchondral bone (underlying cartilage) and counteracting joint decline.
Vijoint HCC is a patent filed in Italy, Germany, Spain, France, Great Britain, Portugal, Turkey, Eurasia, the United States, Morocco, Algeria, and China. At the same time, it is patent pending in Canada, Brazil, and Tunisia—a demonstration of Biofarma Group’s constant commitment to creating products and solutions with high innovation content.


Edited by:
Arianna Vanelli: R&D Manager
Stefania Murzilli: Scientific Specialist
Migliore A., Gigliucci G., De Ponti A., Fornasari D., Iolascon G., Magni A., Tarantino U. “Osteoartrosi: percorsi diagnostico terapeutici per la pratica clinica”, Giornale Italiano di Ortopedia e Traumatologia 2020;46:211-222; doi: 10.32050/0390-0134-278
