

Cosmetics are a category of consumer and distribution products, the production of which is always subject to specific guidelines. In fact, beauty products are applied directly to the skin and other sensitive areas. It is, therefore, necessary to ensure that there is no possibility of adverse reactions due to pathogenic organisms, even in the case of prolonged exposure.
More specifically, cosmetics manufacturers are required to comply with the principles of the Good Manufacturing Practice (GMP) Guidelines, set out in the International Organization for Standardization (ISO) DS/EN ISO 22716:2007 standard, which regulates all aspects of the production, control, storage and shipment of cosmetics [2,3,4,5,6]. The main objective of these guidelines is to define criteria for cosmetic products aimed at ensuring the safety and protection of the consumer [2,3,4,7].
According to GMP, cosmetic products do not have to be sterile, but they must not contain harmful organisms or pathogens. Therefore, a low but stable microbiological population that does not interfere with the product's life may be present.

To meet the required conditions, quality control analyses of cosmetics and, in particular, microbiological analyses of raw materials, bulk and finished products, packaging, personnel, equipment and preparation and storage rooms are mandatory.
In addition, manufacturers must provide information to support the microbiological stability of products, which is essential to demonstrate their overall stability over their life cycle.
For this reason, microbiological risk analyses are carried out, which are necessary to identify the presence of pathogens and opportunistic microorganisms. Since these bacteria are very harmful, the manufacturer must assess their presence and meet the regulations for the specific intended use.
However, not only harmful microorganisms but also beneficial ones can be studied. In fact, in the last period, the cosmetics production market has seen the emergence of a new trend: the use of probiotics or good bacteria in the live state or as ferments or lysates as ingredients in cosmetic products.
In this case, the presence of microorganisms in the formulation of the cosmetic is essential; therefore, the quality control assessment will focus on other aspects, such as the characterization of their viability.
Today, the plate counting method is the reference procedure in evaluating microbiological contaminants and the quality control of cosmetics. The samples to be analyzed are incubated on different media and under other conditions, according to ISO or Harmonized Pharmacopoeia methods. The count on agar plates determines the number of viable pathogens present.
However, this approach requires several days of work and a high amount of bacteria to assess correctly and is highly dependent on the operator. Due to this, the test can be performed much less frequently and only applies to some stages of production, causing limitations to cosmetic quality control procedures.
Precisely for these reasons, the demand for more sensitive methods that allow faster results is increasing from operators in the sector.
The answer to this need can come from molecular technologies, which allow to perform more in-depth and sensitive tests than traditional cultures and therefore represent today one of the frontiers of microbiology [8,9,10,11].
Hence, the study of the Quality Control laboratory of Biofarma Group aimed to develop an innovative method for molecular detection to analyze bacterial DNA as an indicator of the quality of cosmetics.
For Biofarma Group, continuous innovation is synonymous with constant quality. For this reason, it has always been looking for new methods to ensure the release of quality products in compliance with current regulations. The Group boasts a state-of-the-art Quality Control laboratory constantly engaged in research. Among his results, the innovative methods developed for the count of probiotics with the flow cytometer and, precisely, for the search for pathogens in cosmetics using qPCR stand out.
In particular, the main objective of this second study is to provide preliminary evidence on applying a molecular approach to extract and analyze DNA for the quality control of cosmetics, thus circumventing the plate method.
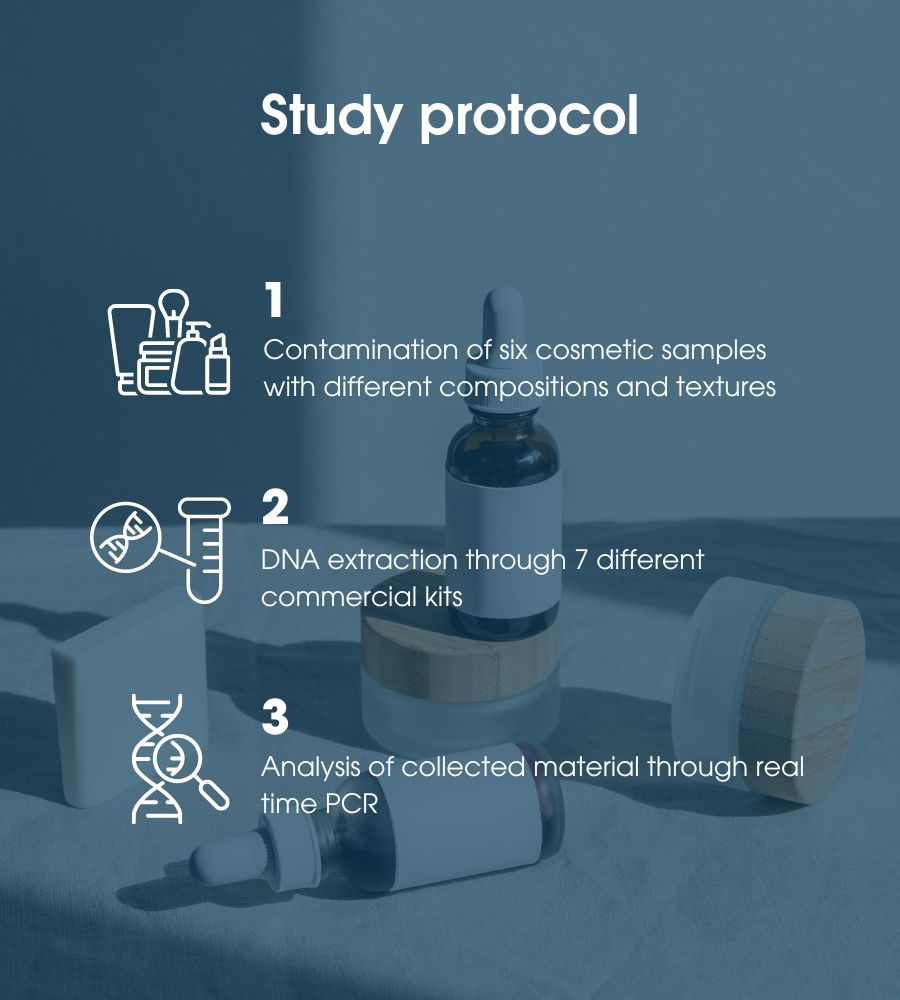
Specifically, during the study, an efficient method for extracting bacterial DNA from cosmetic matrices was first optimized. Subsequently, the potential of the qPCR methodology for the rapid identification and characterization of extracted microbiological contaminants, both probiotics and pathogens, was exploited.
A total of six samples of cosmetics characterized by different compositions of natural ingredients, amino acids, preservatives and other excipients and various physical forms such as aqueous, solid compact, oily, creamy and milky were contaminated for the study.
Three different cell types were used for contamination: the Gram-positive probiotic bacterium Lactobacillus rhamnosus, a commercial strain of Escherichia coli representative of a Gram-negative pathogen, yeast cells Candida albicans.
The DNA was then extracted through the use of seven commercial kits. The seven selected methodologies had the advantage of exploiting different approaches for cell lysis: one kit based on magnetic beads, four kits based on mechanical cell lysis, and two kits based on enzymatic membrane digestion.
The seven methods showed significant differences in the yield and purity of the DNA taken. The highest DNA concentrations resulted from sphere-beating processes, while the lowest results were obtained from membrane enzymatic digestion-based kits.
The collected material was then analyzed by qPCR. This method allows the sequencing of genetic traits and then comparing them with a reference database to identify the presence of pathogens in the researched cosmetic product.
The application of this innovative technology made it possible to obtain detection results in four hours, allowing a rapid assessment of both raw materials sensitive to microbial contamination and probiotics used as biologics, except in the case of Candida albicans for which other intermediate steps will probably be required to increase DNA recovery or to remove qPCR inhibitors.
It is important to specify that DNA-based methods cannot distinguish between living and dead cells unequivocally. Dead cell DNA is indistinguishable from living cell DNA, which can be important to consider in specific contexts. In fact, without selective identification of living microbes, DNA approaches overestimate the types and number of viable microorganisms. Thus, a negative screening result indicates the absence of a specific pathogen in a cosmetic sample, in compliance with microbiological limits. On the other hand, a positive screening result requires further investigations with different techniques to ascertain the viability of the identified cells.
In conclusion, the results obtained from the study confirm that, combined with mechanical extraction, qPCR can represent an efficient and straightforward method for identifying microorganisms in cosmetics. This approach also applies to detecting probiotics used as beneficial biological components in products.
Therefore, the results of this molecular method provided preliminary evidence for the rapid identification of cells (10–100) and nucleic acids in complex preparations used for human health, within regulatory limits. The suggested methodology is a novelty in cosmetics quality control: it is simple, fast and sensitive, and its scalability allows serial microbiological evaluation at every production stage.

The statement of Emanuele Nencioni, QC Manager of Biofarma Group:
"Innovation in Biofarma takes place in many fields, not only in the production of supplements or cosmetics, but also in the continuous search for increasingly high-performance and innovative analysis methods.
For many years now, in the QC laboratory of Biofarma, at the Mereto di Tomba site, I have set up a small research group that devotes passion, curiosity, scientific knowledge and a lot of creativity to developing skills for new analytical methods. Many things have been accomplished in recent years:
Introduction of innovative, state-of-the-art instruments in the QC laboratory (Cytofluorometer, Real Time PCR);
Many publications in scientific journals to consolidate and accredit our activities at international level.
But we are a group always on the lookout for new 'fields' still unexplored because we firmly believe in research and continuous scientific progress."
Edited by:
Emanuele Nencioni, QC Manager
Michelutti, L.; Bulfoni, M.; Bolzon, V.; Nencioni, E. Preliminary Evidence of a Molecular Detection Method to Analyze Bacterial DNA as a Quality Indicator in Cosmetics. Cosmetics 2020, 7, 54.
Iso 22716:2007(en) Cosmetics—Good Manufacturing Practices (GMP)—Guidelines on Good Manufacturing Practices. 2007.
Iso 29621:2017(en) Cosmetics—Microbiology—Guidelines for the Risk Assessment and Identification of Microbiologically Low-Risk Products. 2017
Jaccard, M. The Objective Is Quality, 1st ed.; EPFL Press: Lausanne, Switzerland, 2013
European Pharmacopoeia 7.0. In 5.1.3. Efficacy of Antimicrobial Preservation.
Barel, A.O.; Paye, M.; Maibach, H.I. Handbook of Cosmetic Science and Technology Fourth Edition 2005
Regulation (EC) no 1223/2009 of the European Parliament and the Council of 30 November 2009 on cosmetic products (text with EEA relevance). 2009.
Murakami, S.; Shimamoto, T.; Nagano, H.; Tsuruno, M.; Okuhara, H.; Hatanaka, H.; Funato, K. Producing human ceramide-NS by metabolic engineering using yeast Saccharomyces cerevisiae. Sci. Rep. 2015, 5, 16319
Ricchi, M.; Bertasio, C.; Boniotti, M.B.; Vicari, N.; Russo, S.; Tilola, M.; Bellotti, M.A.; Bertasi, B. Comparison among the quantification of bacterial pathogens by qPCR, dPCR, and cultural methods. Front. Microbiol. 2017, 8, 1174
Versalovic, J.; Lupski, J.R. Molecular detection and genotyping of pathogens: More accurate and rapid answers. Trends Microbiol. 2002, 10, s15–s21
Tsalik, E.L.; Bonomo, R.A.; Fowler, V.G., Jr. New molecular diagnostic approaches to bacterial infections and antibacterial resistance. Annu. Rev. Med. 2018, 69, 379–394.