

With a global prevalence of 10 to 20% and an estimated incidence of around 1.5%, irritable bowel syndrome is the most common functional gastrointestinal disease.
The condition appears to involve women more than men, with onset typically seen between 20 and 50.
The consequences of the disease are numerous: those who experience it suffer a severe reduction in the quality of life, which often leads to a loss of working days, second only to the flu syndrome. Added to this is a significant economic impact regarding medical checkups, diagnostic tests and even often unnecessary surgery.
It is, therefore, a disabling pathology that involves the need, on the part of those who suffer from it, to seek solutions that represent daily support for bringing back the quality of life to an average level.
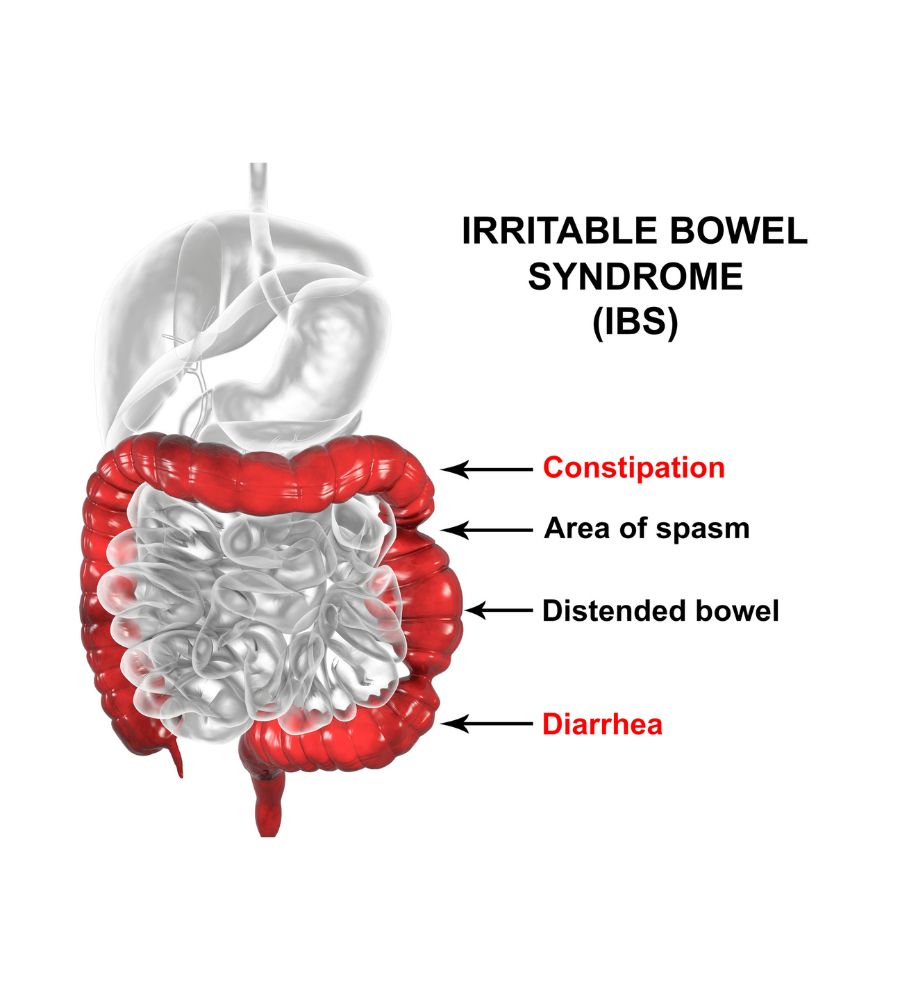
What are the symptoms of irritable bowel? The disease typically presents with abdominal discomfort or pain associated with a change in the frequency of evacuations and an alteration in the appearance of the stool. In addition, mucus and swelling or distension of the abdomen are reported, often alternating. Finally, in addition to these intestinal disorders, most patients experience a feeling of weakness and fatigue.
The causes of irritable bowel syndrome can be numerous but difficult to identify clearly. Those most commonly associated with the condition are physiological, such as, for example, impaired visceral secretion and hypersensitivity, motor alterations, gastrointestinal infections, inflammation of the intestinal mucosa and genetic susceptibility. However, the causes can also be psychosocial, as in the case of problems related to cognitive and emotional aspects.
In this regard, the intestinal microbiota plays a significant role, which can interact with the intestinal mucosa and the body by performing essential structural, metabolic and protective functions. Still, an altered functionality has been observed in the case of irritable bowel syndrome.
Treatment for irritable bowel conditions generally aims at eliminating symptoms, especially considering the difficulty in identifying a specific cause. Treatment may include the use of medicines such, for example, laxatives or prokinetics, non-absorbable antibiotics or intestinal anti-inflammatories.
In addition, the aim is often to educate the patient on adequate nutrition. In fact, as a remedy for the disease, some studies investigate the correlation between nutrition and irritable bowel in addition to the intake of medicines prescribed by doctors. It is often recommended to reduce the consumption of foods that tend to form gas in the intestine, especially if the main symptom is bloating with abdominal distension; In this case, it is advisable to reduce the consumption of carbonated drinks, vegetables, legumes, fruit after meals and, finally, chewing gum. In addition, some studies are investigating the effects of a diet low in oligosaccharides, disaccharides, monosaccharides, and fermentable polyols (FODMAPs) to counteract irritable bowel symptoms.
Finally, patients often resort to the use of nutraceuticals. The beneficial effects of these compounds on human health have emerged significantly in recent decades, making them, together with herbal or vitamin products, generally accepted as safe adjuvant treatments to conventional therapies. This is also confirmed for treating irritable bowel syndrome, against which some supplements have shown essential results: this is the case of the nutraceutical product IBS developed by Biofarma Group.
The Research and Development laboratories of Biofarma group have developed the IBS product: a supplement intended for treating intestinal disorders associated with different degrees of inflammation of the colon.
The innovation of the product is given by the presence of specific active ingredients, supported by scientific evidence, and by technology with the immediate release of the active ingredients in the colon better to direct the therapeutic effects at the site of application.
The main ingredients of the formulation are L-tryptophan, pomegranate titrated in ellagic acid and inulin.
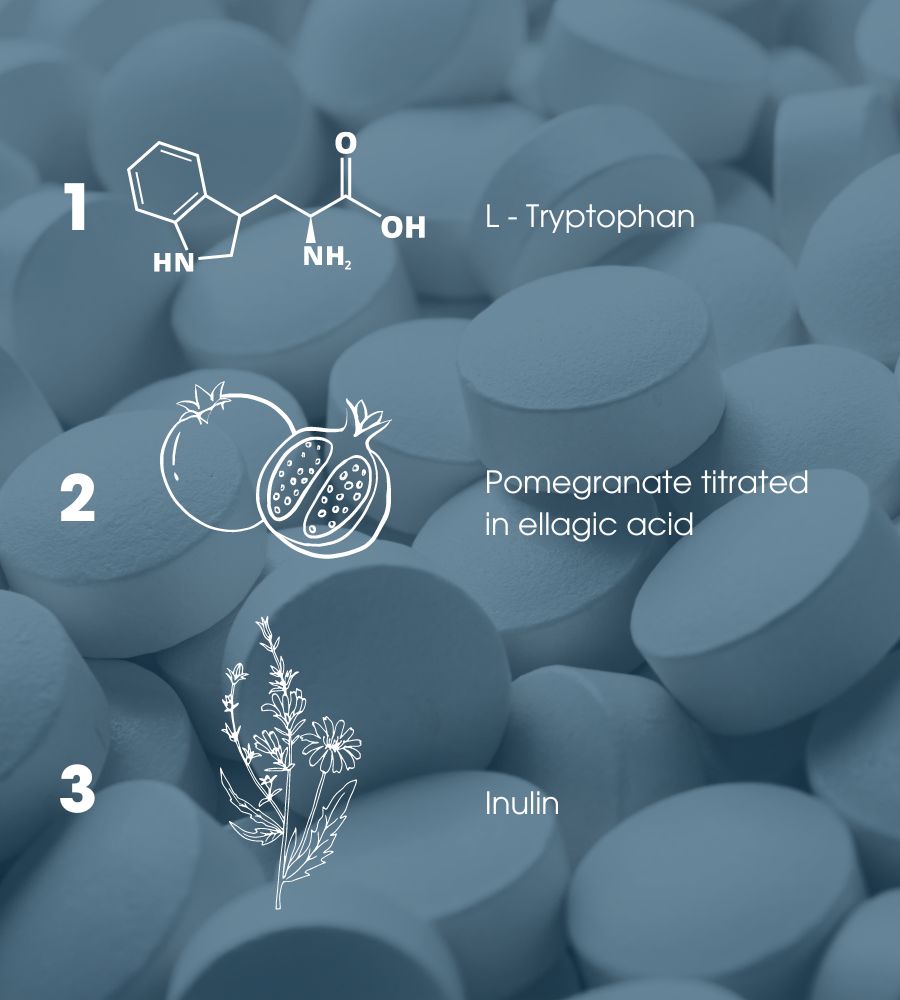
The dietary polyphenols present in Punica granatum (pomegranate), such as ellagic acid, have been shown to exert anti-inflammatory and antioxidant properties. A solid evidence base suggests that pomegranate fruit exerts anti-inflammatory effects that can alleviate symptoms of inflammatory bowel disease and reduce damage to colon tissue and oxidation. In addition, the component is associated with benefits on the regularity of intestinal transit and the general functionality of the digestive tract (Indications authorized according to Ministerial Decree 9/2012 of the Ministry of Health).
Inulin instead, and in particular chicory inulin, has been included as it contributes to normal intestinal function, increasing the frequency of faecal expulsion (EFSA authorized claim according to Art. 13.1 – Regulation 1924/2006:).
In particular, Fibruline® was used for this formulation, a registered trademark of inulin; Specifically, it is a range of chicory root fibres (soluble dietary fibres) extracted by a natural process.
The small intestine does not digest these fibres, and they arrive intact in the colon, where they are fermented totally and selectively by the intestinal microflora, along with the production of short-chain fatty acids (SCFAs). SCFAs improve gut health through a range of local effects, from maintaining intestinal barrier integrity, mucus production and protection against inflammation to reducing the risk of colorectal cancer (Dalile B, 2019).
An intake of 5 g of Fibruline® corresponds to the recommended daily dose of inulin (25 and 30 grams) to have a prebiotic effect.
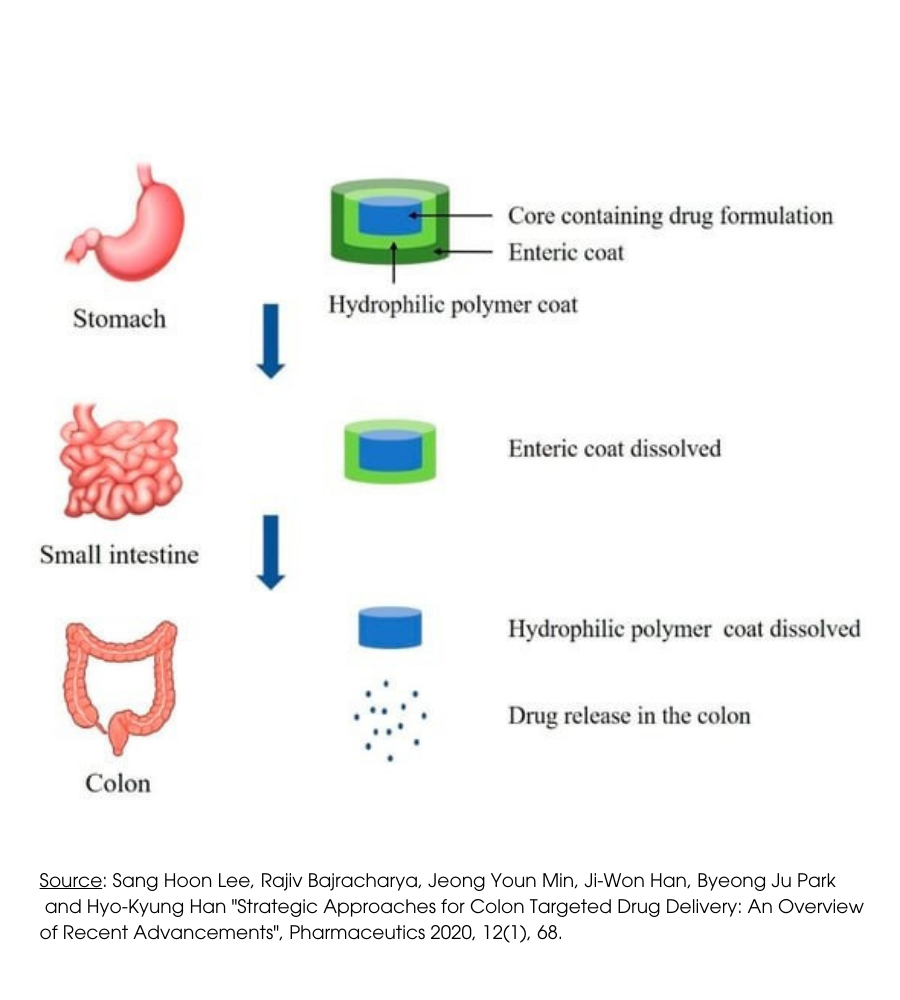
As mentioned, the product’s effectiveness is also due to the delivery technology used: the colon-specific drug delivery system (CDDS).
It is a technology that can protect the drug during the journey to the colon; In this way, the release and absorption of the drug do not take place in the stomach and small intestine and the bioactive agent is not degraded at either site of dissolution but is released and absorbed only when it reaches the colon.
This is especially important as the colon is believed to be a suitable absorption site for peptide and protein drugs for the following reasons:
a lower diversity and intensity of digestive enzymes;
the comparative proteolytic activity of the colon mucosa is much lower than that observed in the small intestine; thus, CDDS protects peptide drugs from hydrolysis and enzymatic degradation in the duodenum and jejunum, and eventually releases the drug into the ileum or colon, which leads to increased systemic bioavailability;
the colon has a long residence time, up to 5 days, and is highly reactive to absorption stimulators.
It is a pharmaceutical technology that Biofarma group has also managed to apply to nutraceuticals, specifically designed for the treatment of those diseases, such as irritable bowel and diverticulosis, where the effectiveness of the active ingredients is greater if released into the mucous membrane of the inflamed colon.
The IBS product’s effectiveness, technology, and components were observed through a preclinical test whose results showed a significant anti-inflammatory effect. The formulation has demonstrated an ability to significantly inhibit acute intestinal inflammation, thus positioning itself as a suitable product for treating irritable bowel.
Edited by:
Antonella Venuti: R&D Manager
Arianna Vanelli: R&D Manager
Stefania Murzilli: Scientific Specialist
Valentina Milite: R&D Food Supplement
Murzilli S., D’Alessio S., Lamberti S., Vanelli A., “The Nutraceutical Approach in the Treatment of Irritable Bowel Syndrome (IBS)”, Biomed J Sci & Tech Res 48(1)- 2023. MS.ID.007584.
Maria Cristina Neri, “La sindrome del colon irritabile” Rivista Società Italiana di Medicina Generale n. 4, vol. 26, 2019
Mitchell H., Porter J., Gibson P. R., Barrett J., Garg M., “Review article: implementation of a diet low in FODMAPs for patients with irritable bowel syndrome-directions for future research”, Aliment Pharmacol Ther. 2019 Jan;49(2):124-139. doi: 10.1111/apt.15079.
